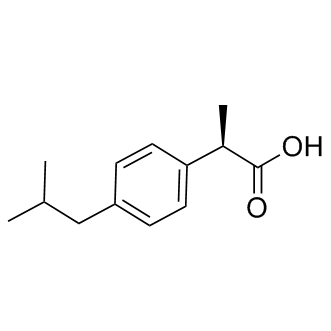
Chemical Structure : (R)-(-)-Ibuprofen
CAS No.:
51146-57-7
(R)-(-)-Ibuprofen
((R)-Ibuprofen)
Catalog No.: PC-45990Not For Human Use, Lab Use Only.
An enantiomer of Ibuprofen that is generally not considered a COX inhibitor and is instead thought to be involved in pathways of lipid metabolism as it is incorporated into triglycerides along with fatty acids.
Bulk size, bulk discount!
Welcome credit card payment!
E-mail: sales@probechem.com
Tech Support: tech@probechem.com
Purity & Documentation Purity:
>98% (HPLC)
Biological Activity
An enantiomer of Ibuprofen that is generally not considered a COX inhibitor and is instead thought to be involved in pathways of lipid metabolism as it is incorporated into triglycerides along with fatty acids; inhibits NF-κB activation (IC50=121.8 uM) in response to T-cell stimulation as well as block superoxide formation, β-glucuronidase release, and LTB4 generation by stimulated neutrophils (IC50 =40-100 uM).
Pain
Approved
Physicochemical Properties
|
M.Wt
|
206.2808
|
|
Formula
|
C13H18O2
|
|
Appearance
|
Solid
|
|
CAS No.
|
|
|
Storage
|
- Solide Powder
-
-20 °C 12 Months; 4°C 6 Months
|
- In Solvent
-
-80 °C 6 Months; -20°C 6 Months
|
|
Shipping
|
|
|
Solubility
|
10 mM in DMSO
|
|
Chemical Name/SMILES
|
Benzeneacetic acid, α-methyl-4-(2-methylpropyl)-,(αR)-
|
References
1. Scheuren N, et al. Br J Pharmacol. 1998 Feb;123(4):645-52.
2. Evans AM. Clin Rheumatol. 2001 Nov;20 Suppl 1:S9-14.
3. Villanueva M, et al. Br J Clin Pharmacol. 1993 Mar;35(3):235-42.
Contact Us
sales@probechem.com