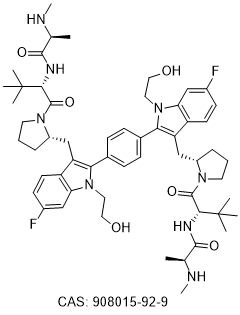
Chemical Structure : IAP antagonist Compound A
CAS No.:
908015-92-9
IAP antagonist Compound A
Catalog No.: PC-23123Not For Human Use, Lab Use Only.
IAP antagonist Compound A is a highly potent, Smac-mimetic IAP antagonist with equivalent binding affinity to XIAP and cIAP1 in the picomolar range, induces rapid degradation of cIAP1, activates NF-κB signaling via inhibtion of cIAP1 and induces TNFα-dependent apoptosis.
Bulk size, bulk discount!
Welcome credit card payment!
E-mail: sales@probechem.com
Tech Support: tech@probechem.com
Purity & Documentation Purity:
>98% (HPLC)
Biological Activity
IAP antagonist Compound A is a highly potent, Smac-mimetic IAP antagonist with equivalent binding affinity to XIAP and cIAP1 in the picomolar range, induces rapid degradation of cIAP1, activates NF-κB signaling via inhibtion of cIAP1 and induces TNFα-dependent apoptosis.
IAP antagonist Compound A competes with Smac for binding to IAPs and also for caspase 9 binding and interacts specifically with the BIR2 and BIR3 domains of cIAP1, cIAP2, and XIAP.
IAP antagonist Compound A (5 nM and 500 nM) exhibits significant killing of Kym1, OVCAR4, and SKOV3 lines.
IAP antagonist Compound A induces rapid degradation of cIAP1 in a binding- and proteasomal-dependent manner.
IAP antagonist Compound A activates NF-κB signaling and induces production of TNFα in an NF-κB-dependent manner in drug-sensitive cell lines.
Brefeldin A and Geldanamycin could blocked cell death induced by compound A.
IAP antagonist Compound A sensitized cells to TNFα-induced cell death.
Physicochemical Properties
|
M.Wt
|
995.27
|
|
Formula
|
C56H76F2N8O6
|
|
Appearance
|
Solid
|
|
CAS No.
|
|
|
Storage
|
- Solide Powder
-
-20°C 12 Months; 4°C 6 Months
|
- In Solvent
-
-80°C 6 Months; -20°C 6 Months
|
|
Shipping
|
|
|
Solubility
|
10 mM in DMSO
|
|
Chemical Name/SMILES
|
(2S,2'S)-N,N'-((2S,2'S)-((2S,2'S)-((1,4-phenylenebis(6-fluoro-1-(2-hydroxyethyl)-1H-indole-2,3-diyl))bis(methylene))bis(pyrrolidine-2,1-diyl))bis(3,3-dimethyl-1-oxobutane-1,2-diyl))bis(2-(methylamino)propanamide)
|
References
1. Vince JE, et al. Cell. 2007 Nov 16;131(4):682-93.
Contact Us
sales@probechem.com